

Our Investigational Product
TU-100 Clinical Trials
We have set up a system that combines the technology and know-how developed in the course of our Japanese Kampo and herbal medicine business, the latest clinical data from Japan, along with development facilities in the US, to further study and potentially obtain approval and market Daikenchuto (TU-100) as a prescription drug in the US. With POI as the field of application, we have formed an advisory team in Japan and the US to advance its development, and are presently conducting late phase II clinical trials.
About TU-100
Overview
- Traditional herbal medicine of Japan (called KAMPO), originally described in “Jin kui yao lue,” textbook of ancient China in the 3rd century
- Consists of three botanical components: Zanthoxylum fruit (Japanese pepper), processed ginger, and ginseng
- Has been used for a variety of GI disorders since its approval in Japan in 1986
- Dosage form: Dried-extract granules


Zanthoxylum fruit
(Japanese pepper)


Processed Ginger


Ginseng


Add the three medicinal plants to water and
extract the ingredients as shown above


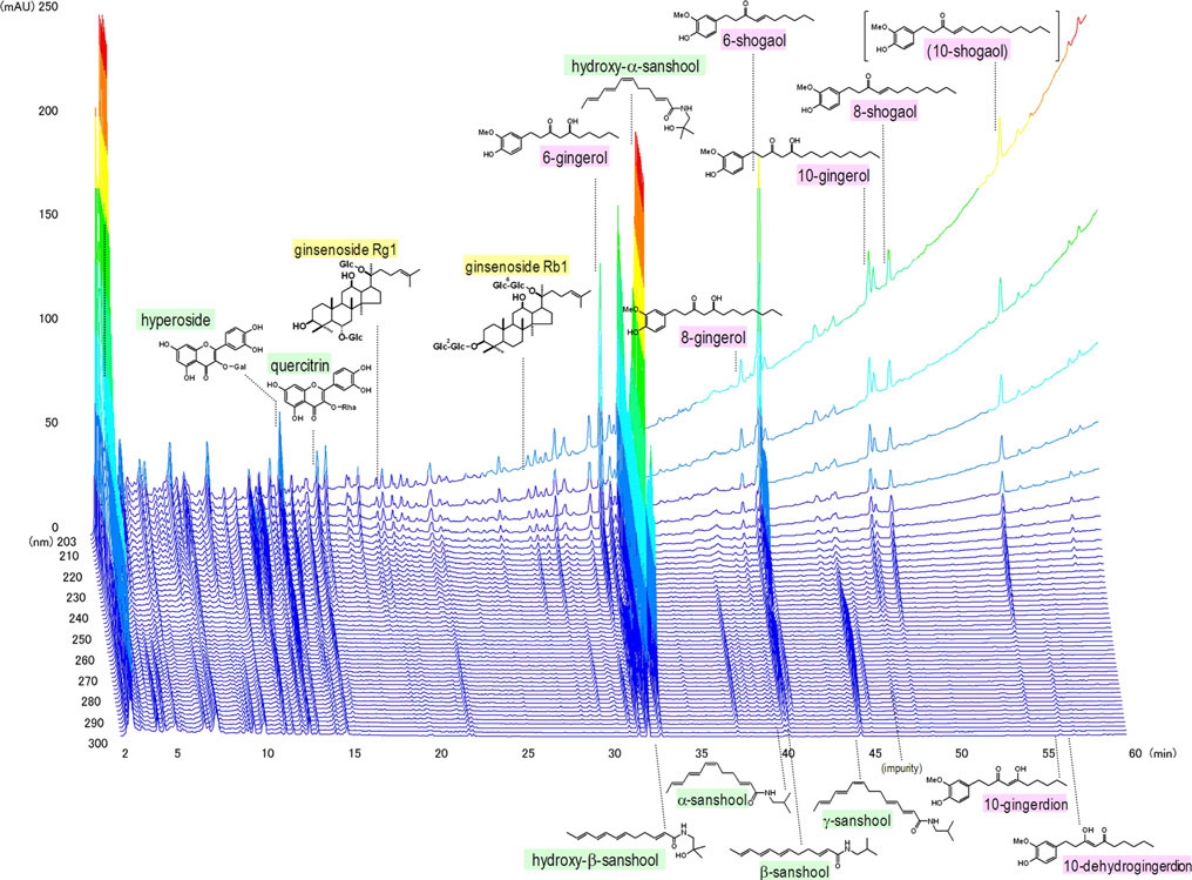
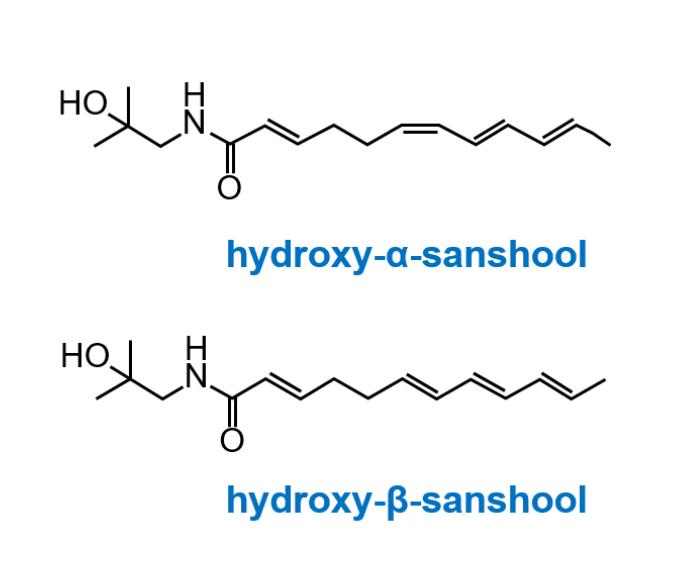
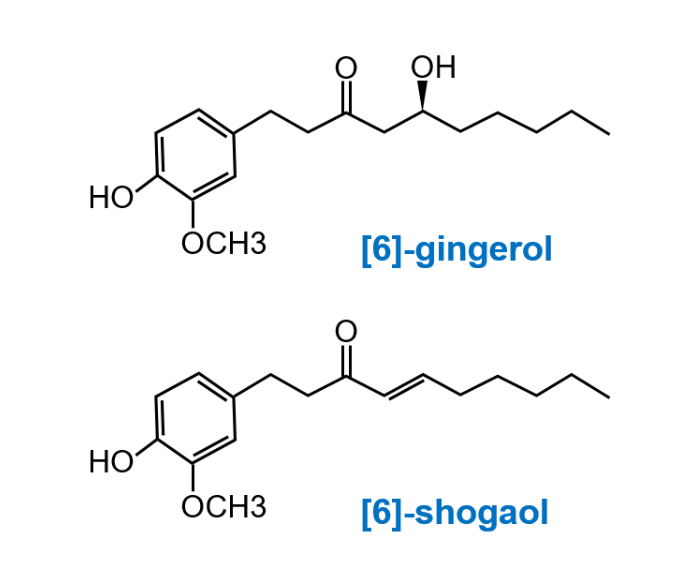
3D-HPLC analysis of TU-100
Kono T, et al. J Gastroenterol. 2011;46:1187–1196.
Quality management control is strictly performed by physical and chemical methods; e.g. three-dimensional high-performance liquid chromatograph (3D-HPLC)#.
#: Fingerprint technology simultaneously evaluates the content of various ingredients in TU-100 as a chromatographic profile. Fingerprints are also required internationally by the US FDA’s Guidance 2016 for Industry Botanical Drug Products.
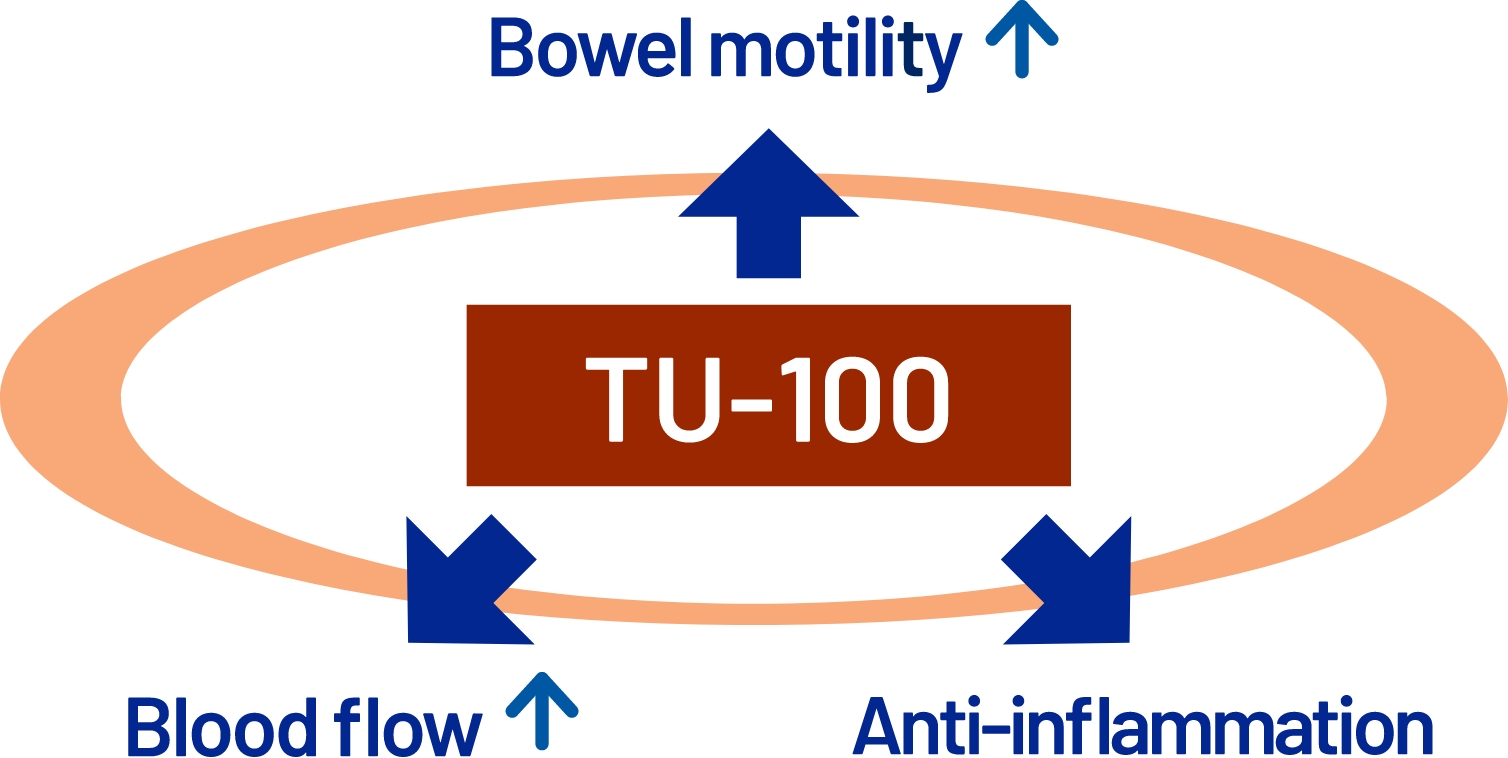
TU-100: three proposed mechanisms of action
Watch a video to learn more
Bibliography
Review
- 1.Ishizuka M, et al. Perioperative administration of traditional Japanese herbal medicine Daikenchuto relieves postoperative ileus in patients undergoing surgery for gastrointestinal cancer: A systematic review and meta-analysis. Anticancer Res. 2017;37(11):5967-74.
- 2.Aoyama T, et al. The clinical effect of kampo medicine in multimodal treatment for Gastrointestinal Cancer in Japan. J Cancer. 2020;11(18):5390-4.
- 3.Kono T, et al. Exodus of kampo, traditional Japanese medicine, from the complementary and alternative medicines: is it time yet? Surgery. 2009;146(5):837-40.
- 4.Toru Kono, et al. Complementary and synergistic therapeutic effects of compounds found in kampo medicine: analysis of daikenchuto. Front Pharmacol. 2015;6:159.
Clinical Studies
- 5.Yoshikawa K, et al. The effects of the kampo medicine (Japanese herbal medicine) “Daikenchuto” on the surgical inflammatory response following laparoscopic colorectal resection. Surg Today. 2012;42(7):646-51.
- 6.Toru Kono, et al. Daikenchuto accelerates the recovery from prolonged postoperative ileus after open abdominal surgery: a subgroup analysis of three randomized controlled trials. Surg Today. 2019;49(8):704-11.
- 7.Manabe N, et al. Effect of daikenchuto (TU-100) on gastrointestinal and colonic transit in humans. Am J Physiol Gastrointest Liver Physiol. 2010;298(6):G970-5.
- 8.Arita R, et al. Responder analysis of Daikenchuto treatment for constipation in poststroke patients: A subanalysis of a randomized control trial. J Evid Based Integr Med. 2019;24:2515690X19889271.
- 9.Taisuke Jo, et al. Reduction in exacerbation of COPD in patients of advanced age using the Japanese kampo medicine Dai-kenchu-to: a retrospective cohort study. Int J Chron Obstruct Pulmon Dis. 2019;14:129-39.
- 10.Suzuki, et al. Daikenchuto increases blood flow in the superior mesenteric artery in humans: A comparison study between four- dimensional phase-contrast vastly undersampled isotropic projection reconstruction magnetic resonance imaging and Doppler ultrasound. PLoS One. 2021;16(1):e0245878.
- 11.Kaido T, et al. Effect of herbal medicine daikenchuto on oral and enteral caloric intake after liver transplantation: A multicenter, randomized controlled trial. Nutrition. 2018;54:68-75.
- 12.Kono T, et al. Distinct effects of TU-100 (daikenchuto) on long-lasting dysbiosis in the small intestine in patients with colorectal cancer and inflammatory bowel disease. Gene. 2022;820:146266.
Safety and Pharmacokinetic Studies
- 13.Iwabu J, et al. Profiling of the compounds absorbed in human plasma and urine after oral administration of a traditional Japanese (kampo) medicine, daikenchuto. Drug Metab Dispos. 2010;38(11):2040-8.
- 14.Munekage M, et al. Pharmacokinetics of daikenchuto, a traditional Japanese medicine (kampo) after single oral administration to healthy Japanese volunteers. Drug Metab Dispos. 2011;39(10):1784-8.
- 15.Munekage M, et al. Population pharmacokinetic analysis of daikenchuto, a traditional Japanese medicine (kampo) in Japanese and US health volunteers. Drug Metab Dispos. 2013;41(6):1256-63.
- 16.Watanabe J, et al. Intestinal, portal, and peripheral profiles of daikenchuto (TU-100)’s active ingredients after oral administration. Pharmacol Res Perspect. 2015;3(5):e00165.
- 17.Katori Y, et al. Adverse drug reaction frequency survey for Tsumura Daikenchuto extract granules (for medical use) (Japanese). Prog Med. 2016;36(4):582-6.
Basic Studies: Increasing Effect on Motility of the Gastrointestinal Tract
- 18.Tokita Y, et al. The pharmacological effects of Daikenchuto, a traditional herbal medicine, on delayed gastrointestinal transit in rat postoperative ileus. J Pharmacol Sci. 2007;104(4):303-10.
- 19. Endo M, et al. Daikenchuto, a traditional Japanese herbal medicine, ameliorates postoperative ileus by anti-inflammatory action through nicotinic acetylcholine receptors. J Gastroenterol. 2014;49(6):1026-39.
- 20.Tsuchiya K, et al. Transient receptor potential ankyrin 1 agonists improve intestinal transit in a murine model of postoperative ileus. Neurogastroenterol Motil. 2016;28(12):1792-805.
- 21.Shibata C, et al. The herbal medicine Dai-Kenchu-Tou stimulates upper gut motility through cholinergic and 5-hydroxytryptamine 3 receptors in conscious dogs. Surgery 1999;126(5):918-24.
- 22.Satoh K, et al. Mechanisms for contractile effect of Dai-kenchu-to in isolated guinea pig ileum. Dig Dis Sci. 2001;46(2):250-6.
- 23.Nakamura T, et al. Abatement of morphine-induced slowing in gastrointestinal transit by Dai-kenchu-to, a traditional Japanese herbal medicine. Jpn J Pharmacol. 2002;88(2):217-21.
- 24.Kubota K, et al. Daikenchuto, a traditional Japanese herbal medicine, promotes colonic transit by inducing a propulsive movement pattern. Neurogastroenterol Motil. 2019;31(11):e13689.
- 25.Kubota K, et al. Hydroxy-α sanshool induces colonic motor activity in rat proximal colon: a possible involvement of KCNK9. Am J Physiol Gastrointest Liver Physiol. 2015;308(7):G579-90.
Basic Studies: Other Unique Effect on the Gastrointestinal Function
- 26.Kono T, et al. Epithelial transient receptor potential ankyrin 1 (TRPA1)-dependent adrenomedullin upregulates blood flow in rat small intestine. Am J Physiol Gastrointest Liver Physiol. 2013;304(4):G428-36.
- 27.Kogure Y, et al. Daikenchuto attenuates visceral pain and suppresses eosinophil infiltration in inflammatory bowel disease in murine models. JGH Open. 2020;4(6):1146-54.
- 28.Wada T, et al. Enhanced anastomotic healing by Daikenchuto (TJ-100) in rats. Sci Rep. 2018;8(1):1091.
- 29.Iwasa T, et al. Feeding administration of Daikenchuto suppresses colitis induced by naive CD4+ T cell transfer into SCID mice. Dig Dis Sci. 2012;57(10):2571-9.
- 30.Kono T, et al. Anti-colitis and -adhesion effects of daikenchuto via endogenous adrenomedullin enhancement in Crohn’s disease mouse model. J Crohns Colitis. 2010;4(2):161-70.
- 31.Hayakawa T, et al. Effects of Dai-kenchu-to on intestinal obstruction following laparotomy. J Smooth Muscle Res. 1999;35(2):47-54.
- 33.Ueno N, et al. TU-100 (Daikenchuto) and ginger ameliorate anti-CD3 antibody induced T cell-mediated murine enteritis: microbe-independent effects involving Akt and NF-κB suppression. PLoS One. 2014;9(5):e97456.
- 34.Kaneko A, et al. Preventive effect of TU-100 on a type-2 model of colitis in mice: Possible involvement of enhancing adrenomedullin in intestinal epithelial cells. Gastroenterol Res Pract. 2013;2013:384057.

